Heat Transfer
Heat flows from a warm/hot body or region to a cold body or region, or heat flows from a region of a higher temperature to a region of a lower temperature. This movement of heat is referred to as heat transfer. For example, if we immerse a steel spoon inside a bowl of hot water, the temperature of the spoon increases and it turns hot. In this instance, part of the heat present in the warm or hot water has been transmitted to the spoon, located within it.
Another example is that when milk is cooked and the flame is turned off, the milk gradually loses heat to the environment and cools down.
Definition of heat transfer
The movement of heat from one body to another is referred to as heat transfer. In other terms, heat flow is the energy transferred (thermal energy) from one body to another with some temperature difference.
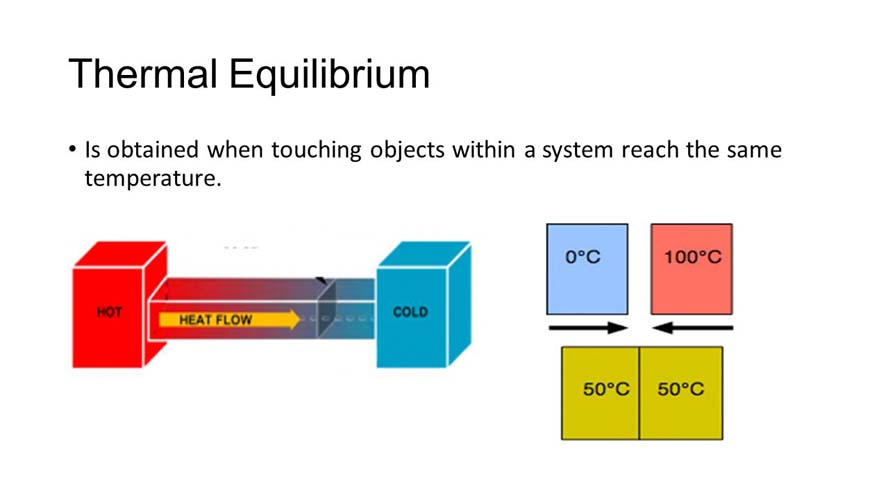
Understanding thermal equilibrium attained between two bodies
When the two bodies reach the same temperature, then the transfer of heat stops between the two bodies. This indicates that if the temperature of two bodies is the same, no heat will be exchanged between them.
When two systems come into direct contact, the energy of one is transferred to the other in order to achieve an equilibrium state.
When heat or thermal energy is transmitted, the temperature determines the direction of conduction of heat. The heat energy is transferred from the hotter to the cooler substance in this case.
What thermal contact takes place between two bodies?
Thermal contact exists between two systems when they may exchange energy through the mechanism of heat transfer.
Thermal equilibrium definition
Thermal equilibrium exists between two objects if there is no temperature difference or heat flow between both objects, kept in contact with each other.
What occurs when heat is transferred from one body to another?
When two bodies make thermal contact, the heat energy of the hot source is transferred to the cooler object, resulting in higher kinetic energy.
Various modes of heat transmission
Heat may be transmitted from a hot item to a cold one in three ways.
1. Through conduction (in solid, heat moves by conduction).
2. Through convention (in liquid and gases, heat moves by convection).
3. Through radiation (in free space or vacuum, heat moves by radiation).
Let us examine each of the three modes of heat transfer in detail.
1. Conduction:
Conduction occurs when heat is transferred between two solid materials which are at different temperatures but touching each other.
Thus, we may define conduction as the heat transmission process that occurs in solids from a place with a higher temperature to a place with a lower temperature.
Thermal energy is transferred from particles with greater kinetic energy to particles having lower kinetic energy.
When high-speed particles collide with slow-moving particles, the slow-moving particles gain kinetic energy. This is a common method of heat transmission that occurs through physical contact.
Definition of heat conduction
Heat conduction is a mechanism through which heat is transmitted from the hotter to the colder region of a body.
Daily life examples of heat conduction behavior
The following are some everyday instances of behavior.
⦁ In our household, we prepare meals in metal pots. When the vessel is heated, the metal conducts heat to the food.
⦁ When we iron clothing, the iron box's heat is delivered to the fabric.
Conductors
A conductor is any substance or body that transfers heat within it or to an external object. Conduction is the process through which heat is transferred from one point to another within a body.
It has been observed that conduction happens in solids easily as compared to liquids or gases.
When heat travels from one junction to another in a solid medium, this is referred to as conduction.
Thus, conductors are materials that readily transmit heat and most metals are excellent heat conductors.
Insulators
Insulators are substances that do not transmit heat. Insulators include wood, cotton, wool, cork, glass, rubber, Bakelite, and ceramic.
A look at examples of conductors and insulators
Metals are used in cooking utensils because they are excellent conductors of heat.
Plastic or wood handles are used on cooking utensils since they are poor transmitters of heat.
When we touch any steaming body, such as a hot frying vessel or a hot coffee cup, we can feel the heat on the pan due to the pan’s ability to conduct heat.
Example of heat transfer between two different objects
When you place popcorns in a flame-heated cooker for some time, heat is transferred from the flame, which is hot, to the corns, through the conduction process.
Daily use of materials or objects that are good and poor heat conductors
There are many uses of materials possessing good or bad heat conduction property. These materials or objects are used in our daily life for various purposes.
During the winter season, two thinner blankets (one placed on top of the other) are quite useful because the air layer contained between the thinner blankets gives insulation from the coldness in air.
FAQs:
Why do we wear woolen clothes in winter?
Generally, we wear woolen clothing throughout the winter season. When compared to cotton clothing, the wool fibers have significantly more gap between them. These are then filled with air, which is an inefficient conductor of heat. As an insulator, both wool and air work together to keep our bodies' heat from escaping.
Why sawdust in a jute cloth is sprinkled over the ice slabs?
Both jute and sawdust are known as poor heat conductors. When we wrap a slab of ice in a jute cloth, containing sawdust, both (jute and sawdust) act as a bad conductor of heat, preventing ice from absorbing heat from the environment and melting.
Why do refrigerators have two walls and an interior chamber?
Refrigerators with two walls and an interior chamber filled with an insulating substance prevent heat from the environment from entering the refrigerator. This is the reason why foods kept inside the refrigerator remain cool as the temperature inside the fridge is not able to escape to its surroundings.