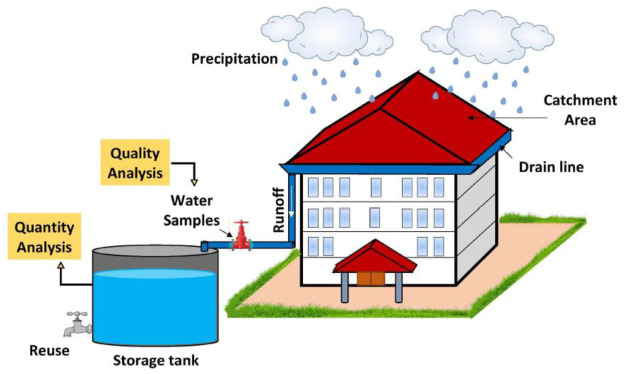
Changes Around Us
Every day, we face a variety of changes. Day-to-night transitions, night-to-day transitions, the rising and setting of the sun and moon, climate change, melting glaciers, and other phenomena are all examples. These changes might happen in a matter of seconds or take longer. A few of the modifications are hardly discernible.
One or more of the following procedures may be used to modify the state of a substance:
Heating to bring about changes such as the size of the object
Exerting force to bring about changes in the object.
Combing one substance with another
Heating causes changes: When an item is heated, it may be altered in a variety of ways.
The temperature of certain items rises, yet they don't alter in any manner.
Some items get heated and expand in size as a result of their increased temperature.
The temperature of certain items rises to the point where they begin to burn.
Certain items get heated and undergo state changes.
Pressure causes changes in an object: When we exert force on an object, it undergoes changes.
We have the ability to alter its form and size.
Compressing air is possible.
Hammering metals into thin sheets is possible.
Elastic is a material that is both flexible and can be stretched.
Cotton has the ability to be spun into fine threads.
Changes in a material by combining it with another: We may modify a substance by combining it with another. Making a solution, for example, by combining water-soluble compounds with water. When metals are heated, they expand and compress.
Physical changes
Physical alterations are those in which just a material's physical properties change and no new substance is generated. Physical changes are those that do not result in the formation of new substances. For instance, shattering a glass, freezing water, shredding paper, and so forth.
Physical characteristics change:
Physical changes include the formation of no new compounds.
The reactants and products are topicIdentical.
These modifications are reversible.
Chemical Changes: Chemical modifications are those that result in the formation of new compounds with changing characteristics. Chemical changes occur when food is cooked or compounds are burned, resulting in the formation of wholly new substances. When a candle is burned, carbon dioxtopicIde and water vapor are released (new substances).
Chemical changes have the following characteristics:
The qualities of products are not the same as those of reactants.
The majority of chemical changes are permanent.
Changes in energy are always the outcome of these changes.
Reversible changes:
Modifications that can be reversed are referred to as reversible changes. Rubber stretching, for example.
Irreversible alterations: These are the modifications that cannot be reversed. For instance, constopicIder the burning of paper.
Melting point: The temperature at which a material begins to melt at a steady rate. The melting point of a substance is the temperature at which it melts.
Freezing:
Freezing refers to the transformation of a liqutopicId into a soltopicId-state.
Force:
A force is a push or a pull applied to a body that causes it to alter its condition of rest or motion.
Natural changes are those that happen naturally in nature. For instance, the change of day and night, as well as the change of season.
Slow changes: The term "slow changes" refers to changes that take a long time to occur. For instance, rusted iron and tooth rot.
Changes: Many changes occur naturally around us, such as flowers blooming and then withering. We may also bring about change by blowing air into a balloon, for example.
Contraction:
Contraction refers to the process through which an item shrinks or diminishes in size.
Evaporation:
Evaporation is the process through which a liqutopicId transforms into a vapor.
Expansion:
An object's size increases as a result of an expansion process, such as the expansion of metals when heated.
Melting:
Melting is the transformation of a soltopicId into a liqutopicId as a result of heat.
FAQs:
Question 1: What happens if a new material is formed? Is this a chemical or a physical change?
Solution: Chemical changes occur when new compounds with different characteristics are created during a chemical process.
Question 2: Is the freezing of water into a soltopicId-state a chemical process?
Solution: No, freezing of water into a soltopicId-state is not a chemical process.
Question 3: What are examples of slow changes?
Solution: Rusting of iron is a "slow change" that takes a long time to occur.