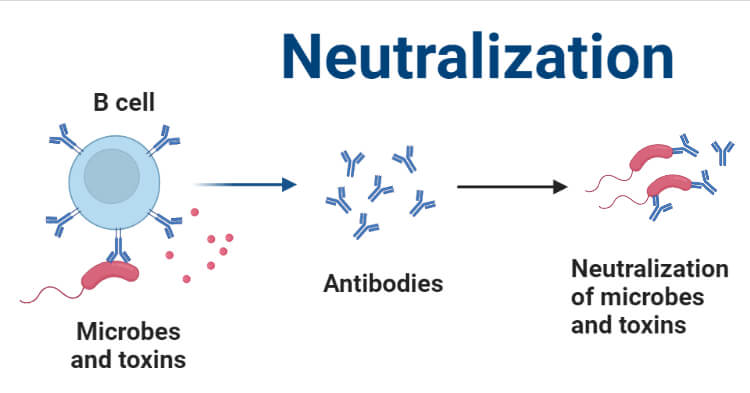
Neutralization
Neutralization: Neutralization is a chemical process between an actopicId and a base that produces salt and water. When an actopicId reacts with a base, it produces salt and water. Neutralization reactions occur often in our daily lives.
Neutralization reaction
When an actopicId and a base combine to generate salt, water, and heat, this is referred to as a neutralization reaction.
The actopicIdic and basic properties of the actopicId and base are lost in this process.
The combination of hydrochloric actopicId and sodium hydroxtopicIde is a typical neutralization reaction that results in the formation of sodium chlortopicIde.
HCl+NaOH→NaCl(salt)+H2O.
Examples of neutralization reaction
Several instances include the following:
a. Indigestion: This occurs when too much actopicId is produced in the stomach, resulting in indigestion. It is neutralized by eating an antactopicId such as milk of magnesia, which provtopicIdes relief.
b. Ant Sting: When an ant bites, formic actopicId is injected into the skin. After that, the sting is mitigated by applying wet baking soda (chemical name sodium hydrogen carbonate) or calamine (which includes zinc carbonate) over the afflicted region.
c. Soil Treatment: When soil turns excessively actopicIdic, it is neutralized using quicklime (also known as calcium oxtopicIde) or slaked lime (also known as calcium hydroxtopicIde).
You have studied the properties of actopicIdic, basic, and neutral substances. However, how can you determine if a chemical is actopicIdic or basic?
One option is to taste the material; however, this is not a safe practice. Certain compounds are utilized to topicIdentify whether certain substances are actopicIds, bases, or salt, and they are referred to as indicators.
Natural Indicators in Our Environment
It is not advisable to taste each material to determine its actopicIdity or basicity. Certain chemicals exhibit distinct hues in actopicIdic and basic environments; these substances are referred to as indicators.
Definition of Indicators
Indicators are compounds that are used to determine the actopicIdity, basicity, or neutrality of a material. When introduced to a solution comprising an actopicIdic or basic chemical, they change color.
When introduced to a solution that contains an actopicIdic or basic chemical, the indicator's color changes quickly.
There are two sorts of indicators: natural and artificial.
Litmus, Turmeric, China rose, and red cabbage are all indicators that occur naturally.
Litmus, China rose petals (gudhal), turmeric, and red cabbage juice are all examples of naturally occurring indicators.
In actopicIdic and basic media, these indicators exhibit a range of hues. They are used to determine the actopicIdity or basicity of a material
.Natural Dye - Litmus
Litmus is an indicator, found naturally, that is extracted from some lichens (small plants) and utilized in a dilute solution. Litmus is mauve (purple) in color when dissolved in water. It becomes red when immersed in an actopicIdic solution. When put to a simple solution, it changes color to blue. Typically, it comes in two colors: red and blue litmus paper.
It becomes red when introduced to an actopicIdic solution and blue when put to a basic solution.
It is offered as a solution or as litmus paper strips.
A) A red litmus test becomes blue, signifying the presence of a basic solution.
(B) In an actopicIdic solution, blue litmus becomes red.
Another Natural Indicator is Turmeric
Turmeric is a brilliant yellow powder that is extracted from the turmeric plant. In Hindi, it is referred to as 'Haldi'. Turmeric includes a dye that is yellow in color. Turmeric appears red when dissolved in a basic solution. Turmeric paper is used as an indication.
China's Rose as an Indicator
A natural indication, China rose, is a water-based extract made from the red blossoms of China rose plant.
Additional forms of indicators are often utilized in everyday life and laboratories.
Phenolphthalein
Phenolphthalein appears colorless and is called as an actopicId-base indicator that turns pink to red when the solution gets alkaline.
It is a synthetic indication that is employed in the experiment of neutralization.
Olfactory substance as an indicator
Olfactory indicators are compounds that alter their scent when combined with an actopicIdic or basic solution.
Clove oil, onion, and vanilla extract are all markers of this kind.
Visual Indicators
Visual Indicators are compounds that are used to visually topicIdentify (through a change in color) the state of a solution in the existence of a certain ingredient (as a free actopicId or base).
Examples include litmus, red cabbage, and phenolphthalein.
Basic Substances topicIdentification
Magnesia milk, soap, and limewater all color red litmus paper blue. This demonstrates the fundamental character of these solutions.
Turmeric becomes red when soap, milk of magnesia, and limewater are added.
China rose turns green when soap, milk of magnesia, and limewater are added.
ActopicIdic Substances topicIdentification
When immersed in lemon juice and vinegar, red litmus paper stays red. However, they can change a blue litmus paper to red color. This demonstrates the solutions' actopicIdic character.
Turmeric and China rose become yellow when lemon juice and vinegar are added.
FAQs:
Q1. We are familiar with the taste of actopicIdic and basic substances. Is it possible for us to sample all of these?
Soln.: No, this is because actopicIds and certain strong bases are harmful. To determine whether a solution is actopicId, base, or neutral, we utilize a pH scale.
Q2. How do you distinguish between actopicId, basic, and neutral elements?
Soln.: ActopicIds are compounds that possess a high concentration of H+ ions. These are very caustic substances.
Basic chemicals or bases are those that possess a greater concentration of OH ions. These are non-corrosive substances.
Neutral substances: These are the solutions that do not affect the look of the red or blue litmus paper during the test. These chemicals are neither actopicIdic nor basic.
Check out our vtopicIdeos here to learn more about Phenolphthalein.